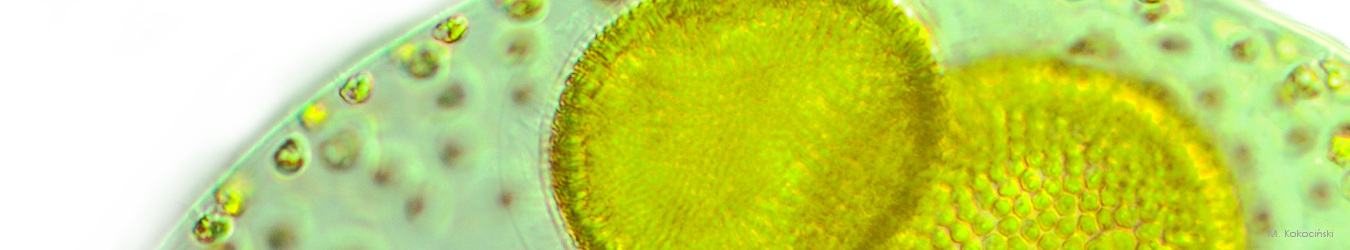
In August 2022, an ecological catastrophe occurred on the Odra River. Low water levels, high temperatures, rapid changes in the salinity of the river due to negative human activities led to massive growth of a harmful alga – Prymnesium parvum. This species releases toxins that are lethal to fish and other gill breathing organisms. Although harmful Prymnesium blooms occur worldwide, this species is still relatively poorly studied.
Below is some basic information about this species.
Prymnesium parvum was first described by Carter in 1937 from Bembridge Pond (Isle of Wight, England) with brackish water. To date, it has been found on islands in the Atlantic, Baltic, and Black Seas, as well as in the Balkans, the United Kingdom, Ireland, Denmark, Finland, Spain, the Netherlands, Germany, Norway, Poland, Portugal, Romania, Russia, Sweden, Ukraine, the United States, Brazil, Israel, China, Australia, and New Zealand, and in North Africa (Morocco) (Algaebase: AlgaeBase :: Listing the World’s Algae, own information).
Morphology: Prymnesium parvum is a unicellular alga of microscopic size. It is 8-16 µm long and 4-10 µm wide. The cells are elongated, irregularly shaped. Two flagella emerging from the furrow are located subapically. These flagella allow the cell to move efficiently. The third structure, located between the flagella, is the haptonema, which serves to attach the cell to the substrate. The haptonema is rigid compared to the flagella and is shorter than the flagella or as long as the flagella. The cells of Prymnesium parvum have two chloroplasts, which have a brown-gold colour due to the dye fucoxanthin. For this reason, the water takes on a golden colour during the period of mass development, and the species is called a ‘golden alga’. The cells have no outer wall, but are encased in double-layered cellulose structures. Cysts are present as surviving forms. Reproduction is predominantly asexual.
Environmental requirements: a euryhaline, eurythermal species, meaning it tolerates a wide range of salinities (1 to over 35 psu) and temperatures ranging from 2 – 32 ̊C (Watson 2001). It is a cosmopolitan species, occurring on all continents except Antarctica. It is most common in saline (estuaries) or brackish marine waters, but is also found in inland waters, often in polluted (highly saline) waters characterised by high conductivity.
Ecology: P. parvum is a mixotrophic species, meaning it has the ability to photosynthesize (autotrophy), but can also feed on dissolved organic matter, bacteria, or protists (heterotrophy). Prymnesium has the ability to kill and eat species that compete with it or are even predators. Mixotrophy of Prymnesium increases when the availability of inorganic compounds decreases (Roelke et al. 2016).
Most blooms are produced by this species in nutrient-rich waters. Reproduction can be triggered by an increase in nutrient concentrations. Limiting nitrogen or phosphorus may increase toxin production and release. Observations indicate that toxicity of the species does not occur at a pH of about 7 or less, whereas an increase in pH triggers toxicity (Roelke, personal communication). A decrease in algal toxicity quickly leads to the decimation of Prymnesium populations by predators (Roelke et al. 2016).
Factors that cause Prymnesium blooms typically include high temperatures, increased salinity, changes in hydrology, and increased nutrient concentrations. Changes in water hardness, the presence of herbicides, and an increase in pH are also thought to occur (Roelke et al. 2016).
Toxins: Prymnesium parvum produces many compounds: lipopolysaccharides (hemolysins), galactoglycerolipids, polene polyethers, cyclo-amines, reactive oxygen species, dimethylsulfiono-propionate, polyunsaturated fatty acids, fish-killing fatty acid amides, cytotoxic, hemolytic, hepatotoxic, neurotoxic, and antimicrobial compounds (Burkholder 2009, Bertin et al. 20121 and 2012b). The polyketide complexes prymnesin-1 and prymnesin-2 have been isolated from Prymnesium and are potent toxins acting on the gills of fish (ichthyotoxins). They are neurotoxic, hemolytic, and cytotoxic compounds (Manning and La Claire 2010).
The effect of toxicity, once the toxins enter the water, can be enhanced by a number of environmental factors (Bertin et al. 2012 a and b). Some fish toxins are photosensitive and their effects may be reduced at pH 7 or lower.
Prymnesium parvum blooms cause death of gill-breathing organisms: fish, mollusks, tadpoles. A common mode of action of Prymnesium is destruction of selective permeability of cell membranes and disruption of ion regulation in gills. Fish infected with the toxin characteristically have bloody gills that can produce a very thick layer of mucus. They then swim slower and slower, lying down near the bottom, congregating near the shore, at fresh water sources or jumping ashore.
Some bioactive compounds secreted by Prymnesium can also suppress the growth or cell lysis of other algae and cause zooplankton to die or reduce their grazing and reproduction rates (Roelke et al. 2016).
Potential opportunities to mitigate negative impacts caused by the species: the basis should be appropriate management of the reservoir or river environment and reduction of nutrient inputs (nitrogen, phosphorus) and salts (generally all pollutants). Near rivers, it is advisable to leave or create refugia that can serve as refuges for fish from toxic conditions, for example.
In aquaculture, chemical control is done with ammonium sulphate or copper-based algaecides to reduce population size, or potassium permanganate to reduce toxicity. However, care should be taken when using these agents, as the addition of ammonium, for example, can produce un-ionised ammonia that is harmful to fish and other aquatic organisms. Lowering the pH and flushing the water body with uncontaminated water also gives good results. (Roelke et al. 2016).
LITERATURE
Bertin, M.J., P.V. Zimba, K.R. Beauchesne, K.M. Huncik, and P.D.R. Moeller. 2012a. Identification of toxic fatty acid amides isolated from the harmful alga Prymnesium parvum Carter. Harmful Algae, 20: 111–116.
Bertin, M.J., P.V. Zimba, K.R. Beauchesne, K.M. Huncik, and P.D.R. Moeller. 2012b. The contribution of fatty acid amides to Prymnesium parvum Carter toxicity. Harmful Algae, 20: 117-125.
Burkholder, J.M. 2009. Harmful algal blooms. W: Encyclopedia of Inland Waters, Vol. 1 G.E. Likens (Ed.). Elsevier, Oxford: p. 264–285.
Manning, S.R., and J.W. La Claire II. 2010. Prymnesins: toxic metabolites of the golden alga, Prymnesium parvum Carter (Haptophyta). Marine Drugs, 8: 678–704.
Roelke, D.L., A. Barkoh, B.W. Brooks, J.P. Grover, K.D. Hambright, J.W. La Claire II, P.D.R. Moeller, and R. Patino. 2016. A chronicle of a killer alga in the west: ecology, assessment and management of Prymnesium parvum blooms. Hydrobiologia, 764: 29-50.
Roelke, D. L., & Manning, S. R. (2018). Harmful Algal Species Fact Sheet: Prymnesium parvum (Carter) “Golden Algae”. Harmful Algal Blooms: A Compendium Desk Reference, 629-632. Chapter 16g, Book Editor(s): Sandra E. Shumway, JoAnn M. Burkholder, Steve L. Morton.
Watson, S. 2001. Literature Review of the Microalga Prymnesium parvum and Its Associated Toxicity. PWD RP T3200-1158. Texas Parks and Wildlife Department, Austin: 39 p.
Description: Elżbieta Wilk-Woźniak